Research Theme
Development of Synthetic Organic Reactions Catalyzed by Organometallic Complexes and the Application to Functional Materials.
By using transition metal catalysts, we can develop the organometallic reagents and the organometallic complexes which show the reactivities and selectivities different than ones in classical methods. We can also control the reactivities of the reagents and the catalysts precisely by tuning the ligands (organic compounds) on the metals.
We are presuing the development of the new carbon-carbon bond formation reactions which become a basis for synthetic organic reactions by talking advantage of the characteristics of the organometallics complexes which consist of the metals and the organic compounds.
We are also interested in the development of the enviromentally friendly reactions for "Green Chemistry" which is one of the most important tasks for chemists in the 21th century.
Reserch Subjects (Click to see details)
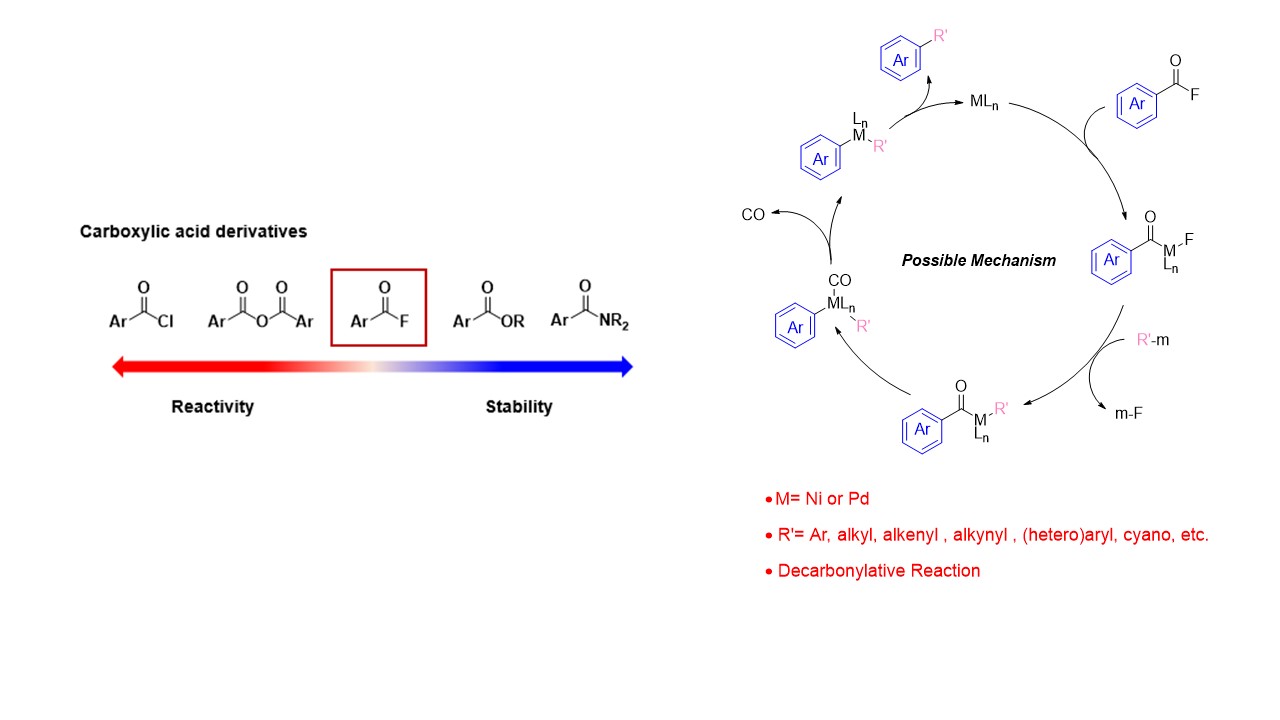
1. Nickel- or Palladium-catalyzed Decarbonylative Transformation of Acyl Fluoride
Although acyl fluorides are easily prepared from the corresponding carboxylic acids, little research has been done on the reactivity of acyl fluorides so far. In order to develop transformation of acyl fluoride, we have suceeded the development of nickel- or palladium-catalyzed decarbonylative carbon-carbon and carbon-hetero atom bond formation reaction of acyl fluoride. These reactions may proceed through oxidative addition, transmetallation, and reductive elimination, and we utilize the isolation of intermediates and computational chemistry to clarify the stage at which the key decarbonylation reaction proceeds.
-
Yasuhiro Okuda, Jie Xu, Takumi Ishida, Chen-an Wang, and Yasushi Nishihara
Nickel-Catalyzed Decarbonylative Alkylation of Aroyl Fluorides Assisted by Lewis-Acidic Organoboranes
ACS Omega 3, 13129-13140 (2018).
- Zhenhua Wang, Xiu Wang, and Yasushi Nishihara
Nickel-catalysed decarbonylative borylation of aroyl fluorides
Chem. Commun. 13969-13972 (2018).
- Xiu Wang, Zhenhua Wang, Yuya Asanuma, and Yasushi Nishihara
Synthesis of 2-Substituted Propenes by Bidentate Phosphine-Assisted Methylenation of Acyl Fluorides and Acyl Chlorides with AlMe3
Org. Lett. 21, 3640-3643 (2019).
- Xiu Wang, Zhenhua Wang, Li Liu, Yuya Asanuma, and Yasushi Nishihara
Nickel-Catalyzed Decarbonylative Stannylation of Acyl Fluorides under Ligand-Free Conditions
Molecules 24, 1671 (2019).
- Zhenhua Wang, Xiu Wang, and Yasushi Nishihara
PPh3‐Assisted Esterification of Acyl Fluorides with Ethers via C(sp3)-O Bond Cleavage Accelerated by TBAT
Catalysts 9, 574 (2019).
- Zhenhua Wang, Xiu Wang, Yasuyuki Ura, and Yasushi Nishihara
Nickel-Catalyzed Decarbonylative Cyanation of Acyl Chlorides
Org. Lett. 21, 6779-6784 (2019).
- Xiu Wang, Zhenhua Wang, and Yasushi Nishihara
Nickel/copper-cocatalyzed decarbonylative silylation of acyl fluorides
Chem. Commun. 55, 10507-10510 (2019).
- Zhenhua Wang, Xiu Wang, and Yasushi Nishihara
Nickel or Palladium-Catalyzed Decarbonylative Transformations of Carboxylic Acid Derivatives
Chem. Asian J. 15, 1234-1247 (2020).
- Liyan Fu, Qiang Chen, Zhenhua Wang, and Yasushi Nishihara
Palladium-Catalyzed Decarbonylative Alkylation of Acyl Fluorides
Org. Lett. 22, 2350-2353 (2020).
- Xiu Wang, Zhenhua Wang, Takumi Ishida, and Yasushi Nishihara
Methoxylation of Acyl Fluorides with Tris(2,4,6-trimethoxyphenyl)phosphine via C-OMe Bond Cleavage under Metal-Free Conditions
J. Org. Chem. 85, 7526-7533 (2020).
- Qiang Chen, Liyan Fu, and Yasushi Nishihara
Palladium/copper-cocatalyzed decarbonylative alkynylation of acyl fluorides with alkynylsilanes: Synthesis of unsymmetrical diarylethynes
Chem. Commun. 56, 7977-7980 (2020).
- Liyan Fu, Qiang Chen, and Yasushi Nishihara
Decarboxylative Cross-Coupling of Acyl Fluorides with Potassium Perfluorobenzoates
Org. Lett. 22, 6388-6393 (2020).
- Liyan Fu, Jingwen You, and Yasushi Nishihara
Palladium-catalyzed decarbonylative and decarboxylative cross-coupling of acyl chlorides with potassium perfluorobenzoates affording unsymmetrical biaryls
Chem. Commun. 57, 3696-3699 (2021).
- Liyan Fu, Qiang Chen, and Yasushi Nishihara
Recent Advances in Transition-metal-catalyzed C-C Bond Formation via C(sp2)-F Bond Cleavage
Chem. Rec. 21, 3394- 3410 (2021).
- Jingwen You, Qiang Chen, and Yasushi Nishihara
Nickel-Catalyzed Decarbonylative Thioetherification of Acyl Fluorides via C-F Bond Activation
Synthesis 53, 3045-3050 (2021).
- Qiang Chen, Liyan Fu, Jingwen You, and Yasushi Nishihara
Ni-Catalyzed Decarbonylative Alkynylation of Acyl Fluorides with Terminal Alkynes under Copper-Free Conditions
Synlett 32, 1560-1564 (2021).
- Qiang Chen, Zhenyao Li, and Yasushi Nishihara
Palladium/Copper-Cocatalyzed Arylsilylation of Internal Alkynes with Acyl Fluorides and Silylboranes: Synthesis of Tetrasubstituted Alkenylsilanes by Three-Component
Org. Lett. 24, 385-389 (2022).
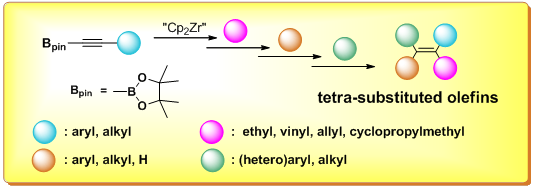
2. Highly regio- and stereoselective synthesis of multi-substituted olefins
Selective synthesis of multi-substituted olefins, so far, is still a challenging work for organic chemists because of its difficulty to control the regio- and stereoselectivity. Our approach involves synthesis of multi-metalated olefins via selective zirconacycle formation of alkynylmetallic reagents and sequential cross-coupling reactions, which can be a good strategy to multi-substituted olefins. We have succeeded in synthesis of several novel multi-arylated and alkylated olefins and are trying to introduce for different aryl groups to the multi-metalated olefins sequentially with high regio- and stereoselectivities.
-
Y. Nishihara, M. Miyasaka, M. Okamoto, H. Takahashi, E. Inoue, K. Tanemura, and K. Takagi
Zirconocene-Mediated Highly Regio- and Stereoselective Synthesis of Multisubstituted Olefins Starting from 1-Alkynylboronates
J. Am. Chem. Soc. 129, 12634-12635 (2007).
- Y. Nishihara, D. Saito, K. Tanemura, S. Noyori, and K. Takagi
Regio- and Stereoselective Synthesis of Multi-Substituted Vinylsilanes via Zirconacycles
Org. Lett. 11, 3546-3549 (2009).
- Y. Nishihara, Y. Okada, J. Jiao, M. Suetsugu, M.-T. Lan, M. Kinoshita, M. Iwasaki, and K. Takagi
Highly Regio- and Stereoselective Synthesis of Multi-alkylated Olefins through Carbozirconation of Alkynylboronates and Sequential Negishi and Suzuki-Miyaura Coupling Reactions
Angew. Chem. Int. Ed. 50, 8660-8664. (2011).
- J. Jiao, Y. Nishihara
Alkynylboron Compounds in Organic Synthesis.
J. Organomet Chem. 721-722, 3-16 (2012).
- Jiao Jiao, Kiyohiko Nakajima, and Yasushi Nishihara
Synthesis of Multisubstituted Olefins through Regio- and Stereoselective Silylborylation of an Alkynylboronate/ Chemoselective Cross-Coupling Sequences
Org. Lett. 15, 3294-3297 (2013)
- Jiao Jiao, Keita Hyodo, Hao Hu, Kiyohiko Nakajima, and Yasushi Nishihara
Selective Synthesis of Multisubstituted Olefins Utilizing gem- and vic-Diborylated Vinylsilanes Prepared by Silylborylation of an Alkynylboronate and Diborylation of Alkynylsilanes
J. Org. Chem. 79, 285-295 (2014).
- Keita Hyodo, Masato Suetsugu, and Yasushi Nishihara
Diborylation of Alkynyl MIDA Boronates and Sequential Chemoselective Suzuki-Miyaura Couplings: A Formal Carboborylation of Alkynes
Org. Lett. 16, 440-443 (2014).
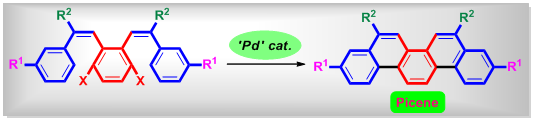
3. Precise Synthesis of Phenacene-Type Organic Molecules
Picene contains a condensed conjugated system with five benzene rings, which shows a high potential utility for organic field-effect transistor (OFET) and organic thin film solar cells. However, there are still few examples of the systematic synthesis of picene and its derivatives. We designed our synthetic methodology, which is more efficient than previous synthetic methods.
-
Ning-hui Chang, Xi-chao Chen, Hikaru Nonobe, Yasuhiro Okuda, Hiroki Mori, Kiyohiko Nakajima, and Yasushi Nishihara
Synthesis of Substituted Picenes through Pd-Catalyzed Cross-Coupling Reaction/Annulation Sequence and Their Physicochemical Properties
Org. Lett. 15, 3558-3561 (2013).
- Ning-hui Chang, Hiroki Mori, Xi-chao Chen, Yasuhiro Okuda, Takeru Okamoto, and Yasushi Nishihara
Synthesis of Substituted [6]Phenacenes through Suzuki-Miyaura Coupling of Polyhalobenzene with Alkenylboronates and Sequential Intramolecular Cyclization via C-H Bond Activation
Chem. Lett. 42, 1257-1259 (2013).
- Yasushi Nishihara, Megumi Kinoshita, Keita Hyodo, Yasuhiro Okuda, Ritsuko Eguchi, Hidenori Goto, Shino Hamao, Yasuhiro Takabayashi, and Yoshihiro Kubozono
Phenanthro[1,2-b:8,7-b']dithiophene: A New Picene-type Molecule for Transistor Applications
RSC Advances 3, 19341-19347 (2013).
- Hiroki Mori, Xi-chao Chen, Ning-hui Chang, Shino Hamao, Yoshihiro Kubozono, Kiyohiko Nakajima, and Yasushi Nishihara
Synthesis of Methoxy-Substituted Picenes: Substitution Position Effect on Their Electronic and Single-Crystal Structures
J. Org. Chem. 79, 4973-4983 (2014).
- Xi-Chao Chen, Shuhei Nishinaga, Yasuhiro Okuda, Jia-Ji Zhao, Jie Xu, Hiroki Mori, and Yasushi Nishihara
A divergent synthesis of 3,10-dialkylpicenes
Org. Chem. Front. 3, 536-541 (2015).
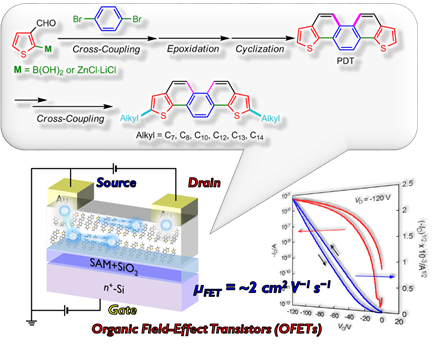
4. Development of Sulfur-Containing Polycyclic Aromatic Hydrocarbons (PAHs) and Their Application to Organic Field-effect transistors
Thiophene-fused polycyclic aromatic hydrocarbons (PAHs) have strong intermolecular interaction and extremely high carrier mobility, because the large atomic radius of sulfur atoms can serve as an effective π-π overlap between the neighboring molecules. Thus, thiophene-fused PAHs are very useful class of compounds for high-performance organic field-effect transistors (OFETs). By using precisive organic synthesis, recently, we have successfully synthesized new phenacene-type thiophene-fused PAHs. In addition, we have developed an efficient and readily scalable synthetic method to afford the large amount of them. OFET devices based on these new compound exhibited high field-effect mobility of up to 2 cm2 V-1 s-1, which are comparable to the conventional amorphous silicon FETs.
-
Yasushi Nishihara, Megumi Kinoshita, Keita Hyodo, Yasuhiro Okuda, Ritsuko Eguchi, Hidenori Goto, Shino Hamao, Yasuhiro Takabayashi, and Yoshihiro Kubozono
Phenanthro[1,2-b:8,7-b']dithiophene: A New Picene-type Molecule for Transistor Applications
RSC Adv. 3, 19341-19347 (2013).
-
Keita Hyodo, Hikaru Nonobe, Shuhei Nishinaga, and Yasushi Nishihara
Synthesis of 2,9-dialkylated phenanthro[1,2-b:8,7-b']dithiophenes via cross-coupling reactions and sequential Lewis acid-catalyzed regioselective cycloaromatization of epoxide
Tetrahedron Lett. 55, 4002-4005 (2014).
-
Yoshihiro Kubozono, Keita Hyodo, Hiroki Mori, Shino Hamao, Hidenori Goto, and Yasushi Nishihara
Transistor application of new picene-type molecules, 2,9-dialkylated phenanthro[1,2-b:8,7-b']dithiophenes
J. Mater. Chem. C 3, 2413-2421 (2015).
-
Yoshihiro Kubozono, Keita Hyodo, Shino Hamao, Yuma Shimo, Hiroki Mori, and Yasushi Nishihara
Transistor Properties of 2,7-Dialkyl-Substituted Phenanthro[2,1-b:7,8-b']dithiophene
Sci. Rep. 6, 38535/1-9 (2016).
-
Keita Hyodo, Hideki Hagiwara, Ryota Toyama, Hiroki Mori, Shin-ichi Soga, and Yasushi Nishihara
Bis[1]benzothieno[2,3-d:2',3'-d']anthra[1,2-b:5,6-b']dithiophene: Synthesis, Characterization, and Application to Organic Field-Effect Transistors
RSC Adv. 7, 6089-6092 (2017).
-
Keita Hyodo, Hideki Hagiwara, Ryota Toyama, Hiroki Mori, Shin-ichi Soga, and Yasushi Nishihara
Synthesis and Physicochemical Properties of Piceno[4,3-b:9,10-b']dithiophene Derivatives and Their Application in Organic Field-Effect Transistors
ACS Omega 2, 308-315 (2017).
-
Shuhei Nishinaga, Hiroki Mori, and Yasushi Nishihara
Synthesis and Transistor Application of Bis[1]benzothieno[6,7-d:6',7'-d']benzo[1,2-b:4,5-b']dithiophenes
J. Org. Chem. 83, 5506-5515 (2018).
-
Shuhei Nishinaga, Hiroki Mori, and Yasushi Nishihara
Synthesis and Transistor Characteristics of Dinaphtho[2,3-d:2',3'-d']anthra[1,2-b:5,6-b']dithiophene (DNADT)
Chem. Lett. 47, 1409-1411 (2018).
-
Keita Hyodo, Shuhei Nishinaga, Yuta Sawanaka, Takumi Ishida, Hiroki Mori, and Yasushi Nishihara
Synthesis and Physicochemical Properties of Dibenzo[2,3-d:2',3'-d']anthra[1,2-b:5,6-b']dithiophene (DBADT) and Its Derivatives: Effect of Substituents on Their Molecular Orientation and Transistor Properties
J. Org. Chem. 84, 698-709 (2019).
-
Shuhei Nishinaga, Masato Mitani, Hiroki Mori, Toshihiro Okamoto, Jun Takeya, and Yasushi Nishihara
Bis[1]benzothieno[5,4-d:5',4'-d']benzo[1,2-b:4,5-b']dithiophene Derivatives: Synthesis and Effect of Sulfur Positions on Their Transistor Properties
Bull. Chem. Soc. Jpn. 92, 1107-1116 (2019).
-
Takumi Ishida, Yuta Sawanaka, Ryota Toyama, Zhenfei Ji, Hiroki Mori, and Yasushi Nishihara
Synthesis of Dinaphtho[2,3-d:2',3'-d']anthra[1,2-b:5,6-b']dithiophene (DNADT) Derivatives: Effect of Alkyl Chains on Transistor Properties
Int. J. Mol. Sci. 21, 2447/1-16 (2020).
-
Zhenfei Ji, Zeliang Cheng, Hiroki Mori, and Yasushi Nishihara
Synthesis and Physicochemical Properties of 2,7-Disubstituted Phenanthro[2,1-b:7,8-b']dithiophenes
Molecules 25, 3842/1-12 (2020).
5. Development of Phenacene-based Semiconducting polymers and Their Application to High-Performance Organic Solar Cells
Thiophene-fused polycyclic aromatic hydrocarbons are also a useful building unit for high-performance semiconducting polymers. Among them, thiophene-fused phenacene-type molecules are promising building unit due to their low-lying HOMO energy levels. However, such building units still limited because of their synthetic difficulty. Recently, we have succeeded the synthesis of new semiconducting polymers containing newly developed phenacene-type molecules. At the present time, the fabricated solar cells using such new phenacene-based polymers exhibited high power conversion efficiency of over 6%.
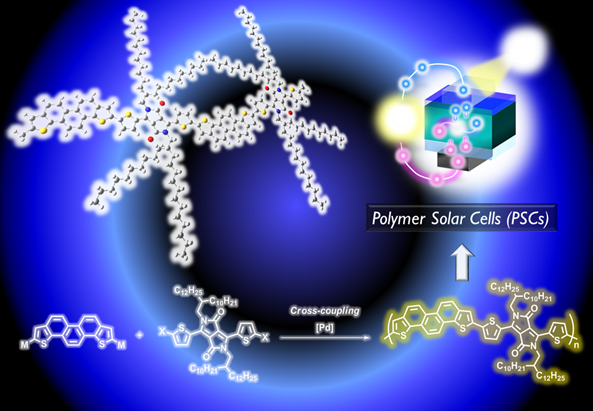
-
Hiroki Mori, Masato Suetsugu, Shuhei Nishinaga, Ning-hui Chang, Hikaru Nonobe, Yasuhiro Okuda, and Yasushi Nishihara
Synthesis, Characterization, and Solar Cell and Transistor Applications of Phenanthro[1,2-b:8,7-b']dithiophene-Diketopyrrolopyrrole Semiconducting Polymers
J. Polym. Sci., Part A: Pol. Chem. 53, 709-718 (2015).
-
Shuhei Nishinaga, Hiroki Mori, and Yasushi Nishihara
Phenanthrodithiophene-Isoindigo Copolymers: Effect of Side Chains on Their Molecular Order and Solar Cell Performance
Macromolecules 48, 2875-2885 (2015).
-
Shuhei Nishinaga, Hiroki Mori, and Yasushi Nishihara
Impact of Alkyl Side Chains on Thin-Film Transistor Performances in Phenanthrodithiophene-Isoindigo Copolymers
Chem. Lett. 44, 998-1000 (2015).
-
Hiroki Mori, Hikaru Nonobe, and Yasushi Nishihara
Highly Crystalline, Low Band-gap Semiconducting Polymers Based on Phenanthrodithiophene-Benzothiadiazole for Solar Cells and Transistors
Polym. Chem. 7, 1549-1558 (2016) (cover picture).
-
Hiroki Mori, Shuto Hara, Shuhei Nishinaga, and Yasushi Nishihara
Solar Cell Performance of Phenanthrodithiophene-Isoindigo Copolymers Depends on Their Thin-Film Structure and Molecular Weight
Macromolecules 50, 4639-4648 (2017).
-
Hiroki Mori, Ryosuke Takahashi, Keita Hyodo, Shuhei Nishinaga, Yuta Sawanaka, and Yasushi Nishihara
Phenanthrodithiophene (PDT)-Difluorobenzothiadiazole (DFBT) Copolymers: Effect on Molecular Orientation and Solar Cell Performance of Alkyl Substitution onto a PDT Core
Macromolecules 51, 1357-1369 (2018).
-
Hiroki Mori, Shuto Hara, Ryota Toyama, Yuya Asanuma, Ryosuke Takahashi, Shuhei Nishinaga, and Yasushi Nishihara
Effect of Substitution Positions of Alkyl Side Chains in Phenanthrodithiophene-Isoindigo Copolymers: The Enhancement of Crystallinity and Control of Molecular Orders
J. Polym. Sci., Part A: Pol. Chem. 56, 1757-1767 (2018).
-
Hiroki Mori, Ryosuke Takahashi, and Yasushi Nishihara
Development of a Phenanthrodithiophene-Difluorobenzoxadiazole Copolymer Exhibiting High Open-Circuit Voltage in Organic Solar Cells
J. Polym. Sci., Part A: Pol. Chem. 56, 2646-2655 (2018).
-
Hiroki Mori and Yasushi Nishihara
Low-Bandgap Semiconducting Polymers Based on Sulfur-Containing Phenacene-Type Molecules for Transistor and Solar Cell Applications
Polymer J. 50, 615-625 (2018).
-
Hiroki Mori
Development of semiconducting polymers based on a novel heteropolycyclic aromatic framework
Polymer J. 53, 975-987 (2021) (cover picture).
6. Development of semiconducting polymers based on novel electron-deficient heteropolycyclic aromatic frameworks
Since heteropolycyclic aromatic compounds including thiadiazole and pyrazine rings have high electron-accepting nature, they are useful building blocks for high-performance p-type and n-type semiconducting polymers. We have successfully synthesized novel electron-deficient heteropolycyclic aromatic compounds containing thiadiazole and pyrazine rings, and their copolymers. The synthesized polymers exhibited good field-effect transistor (FET) and organic photovoltaic (OPV) properties. Therefore, these novel electron-deficient heteropolycyclic aromatic frameworks are potential building blocks for high-performance semiconducting polymers.
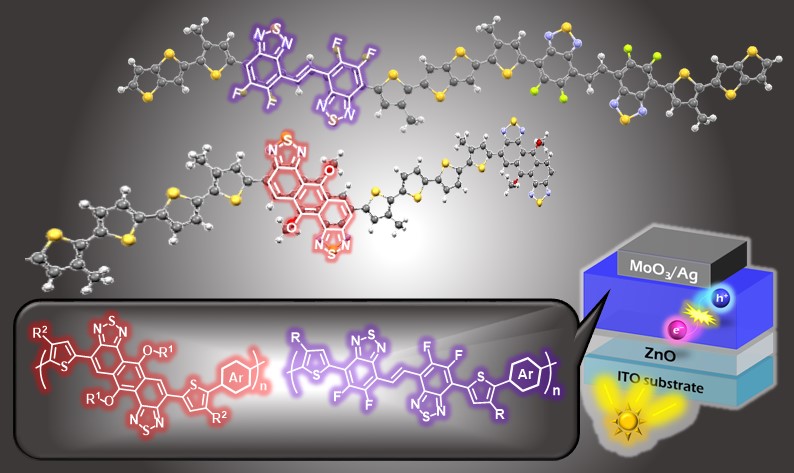
-
Hiroki Mori, Masato Suetsugu, Shuhei Nishinaga, Ning-hui Chang, Hikaru Nonobe, Yasuhiro Okuda, and Yasushi Nishihara
Alkoxy-Substituted Anthra[1,2-c:5,6-c']bis([1,2,5]thiadiazole) (ATz): a New Electron-Acceptor Unit in the Semiconducting Polymers for Organic Electronics
Macromolecules 51, 5473-5484 (2018).
-
Yuya Asanuma, Hiroki Mori, Ryosuke Takahashi, and Yasushi Nishihara
Vinylene-Bridged Difluorobenzo[c][1,2,5]thiadiazole (FBTzE): A New Electron-Deficient Building Block for High-Performance Semiconducting Polymers in Organic Electronics
J. Mater. Chem. C 7, 905-916 (2019).
-
Yuya Asanuma, Hiroki Mori, and Yasushi Nishihara
Transistor Properties of Semiconducting Polymers Based on Vinylene-bridged Difluorobenzo[c][1,2,5]thiadiazole (FBTzE)
Chem. Lett. 48, 1029-1031 (2019).
-
Hiroki Mori
Development of semiconducting polymers based on a novel heteropolycyclic aromatic framework
Polymer J. 53, 975-987 (2021) (cover picture).
Past Research Subject Click here