|
|
Biologically active natural products have been regarded as the promising
drug candidates and useful tool for life science. Particularly, because
of their potent biological activities, natural products isolated from marine
organisms have attracted much attention of organic chemists, biologists,
and pharmacologists. Since further biological studies are hampered by the
limited availability from nature, chemical synthesis has been the sole
realistic way to obtain sufficient amounts of the materials. Furthermore,
due to their unique molecular architectures, these molecules are
particularly attractive targets for synthetic chemists.
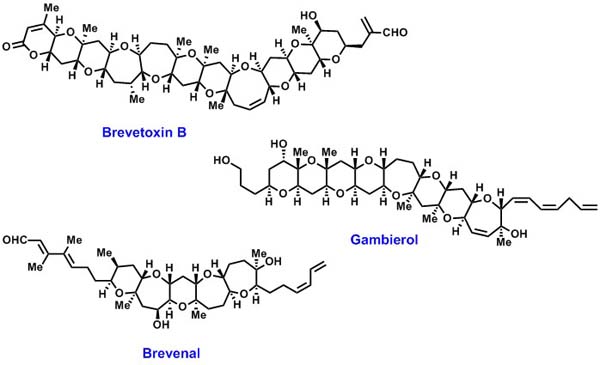
Since the discovery of brevetoxin B in 1981, a number of polycyclic ethers
have been isolated from marine algae. These compounds show potent neurotoxicity
by binding to the ion channels and cause massive fish kills and human food
poisoning. Moreover, the unusual ladder-shaped structures of these
compounds are particularly attractive targets for synthetic chemists. We
have developed an efficient method for the convergent synthesis of polycyclic
ethers via the intramolecular allylation and subsequent ring-closing metathesis.
The methodology has beeb successfully applied to the total synthesis
of marine natural products gambierol, brevetoxin B, and brevenal. Further
studies towards the total synthses of marine polycyclic ethers are in progress. |
| |
 |
| |
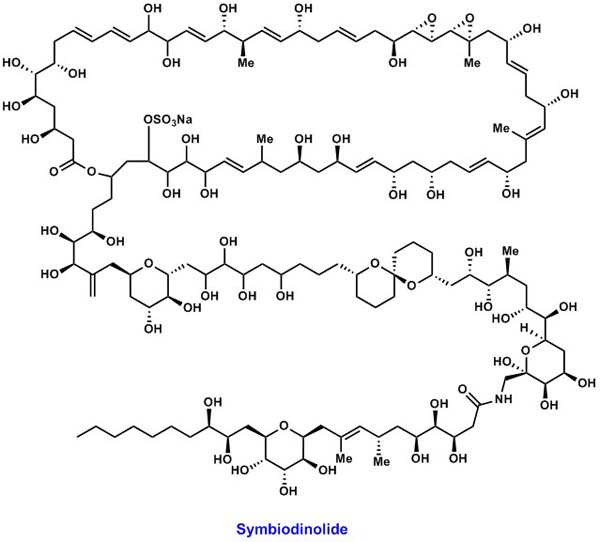
| Structural elucidation of natural products is not only fundamental but
also significant research theme in natural product chemistry. If
the target molecule has a huge molecular size and a number of functional
groups, the chemical synthesis is needed for the configurational assignment.
We have examined the synthetic approach toward the structural
determination of the polyol natural product symbiodinolide. |
| |
 |
| |
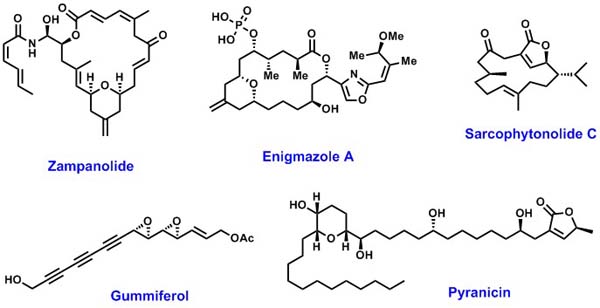
| We are also investigating the development of new synthetic methdology and
its application to the total synthesis of natural products. Selected
examples of the target molecules are shown below. |
| |
 |
| |
| |
| |
| |
|